Abstract
Introduction: The Comprehensive Assessment Tool for Challenges in Hemophilia (CATCH) was developed in 2018 to capture various outcomes that were important to children and adult persons with hemophilia (PwH) and their caregivers. It contains concepts related to quality of life, lifestyle restrictions, and physical activity, and was intended to be used in clinical trial and clinical practice settings across therapies, with adult and pediatric PwH A or B (with or without inhibitors) and caregivers of PwH. In this study, the abbreviated CATCH 2.0 was developed and validated to capture a selected set of outcomes that are most important to PwH and their caregivers.
Methods: This was a psychometric validation study using data from a prospective multi-center single arm trial (that included children and adult PwH with mild to moderate hemophilia A), a US-based psychometric study (that included children and adult PwH A or B), and a nationwide survey of adult PwH A. Item-level analyses assisted in the identification of potential questions for deletion, followed by exploratory factor analysis - which examined relationships between the measured survey items and their domains. Reliability (internal consistency and test-retest), validity (construct, convergent, criterion and known-groups), responsiveness to change, and thresholds for meaningful change for each domain were evaluated.
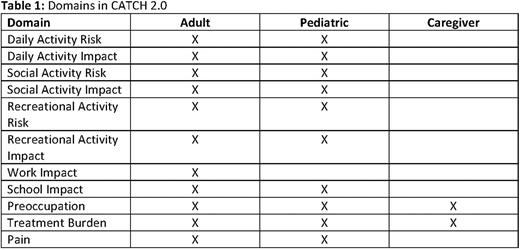
Results: Data from 126 children, 232 adults, and 118 caregivers were analyzed across the 3 studies. This included data from 396 (83%) PwH A and 80 (17%) PwH B, of which 68 (14%) had severe disease and 408 (86%) had mild or moderate hemophilia, and 115 (24%) had active inhibitors. In general, the CATCH 2.0 demonstrated acceptable psychometric properties. Internal consistency reliability for all domains (alpha > 0.87) and test-retest reliability (intraclass correlation coefficient >0.70) were acceptable for 6 of 8 domains. Medium to large correlations (correlation coefficient 0.3 to 0.6) were reported between associated measures (EQ-5D, WPAI, and pain rating) and the majority of CATCH domains. Known-groups validity for most domains (based on categorizations of severity and inhibitor status) was good. Treatment Burden was the only domain which demonstrated any responsiveness over time. Most correlations with annualized bleed rate (ABR) were not significant. Since anchor-based methods using ABR were not feasible (due to poor correlation), thresholds for clinically meaningful change were estimated using a distribution-based approach (standard error of measurement ranged from 6.7 to 16.5). Table 1 contains a list of the domains included in each of the 3 versions. Each version is expected to take < 10 minutes to complete.
Conclusions: The CATCH 2.0, an abbreviated version of the original CATCH, captures important outcomes for PwH A and B, along with their caregivers. CATCH 2.0 has an excellent factor structure and its psychometric properties demonstrated acceptable performance, particularly for reliability, convergent validity, and known-groups validity. Support for responsiveness in mild/moderate populations however, was limited. Results highlight the need for more longitudinal data in heterogeneous groups of PwH to further refine the performance of this tool.
Disclosures
Decker-Palmer:Genentech, Inc.: Current Employment; Roche Holding: Current equity holder in private company, Current holder of stock options in a privately-held company. Mathias:Health Outcomes Solutions (: Current Employment; F. Hoffmann-La Roche Ltd: Research Funding. Colwell:Health Outcome Solutions: Consultancy. Crosby:Health Outcomes Solutions: Consultancy. Gentile:Genentech, Inc: Current Employment. Chupka:Hemophilia Federation of America: Current Employment. Sidonio, Jr.:Novo Nordisk: Honoraria; Bayer: Honoraria; Guardian Therapeutics: Honoraria; Octapharma: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; UniQure: Honoraria; Biomarin: Honoraria; Genentech: Honoraria, Research Funding; Spark: Honoraria; Pfizer: Honoraria. Recht:Oregon Health & Science University: Ended employment in the past 24 months; Catalyst Biosciences, CSL Behring, Genentech, Grifols, Hema Biologics, Novo Nordisk, Pfizer, Sanofi, Takeda, uniQure: Consultancy; Bayer, Biomarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, uniQure: Research Funding; Foundation for Women and Girls with Blood Disorders; Partners in Bleeding Disorders: Thrombosis and Hemostasis Societies of North America: Membership on an entity's Board of Directors or advisory committees; American Thrombosis and Hemostasis Network; Yale University School of Medicine: Current Employment. Shapiro:Genentech, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sangamo Biosciences: Consultancy; Kedrion Biopharma: Research Funding; Sigilon: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioverativ/Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk Hemophilia Foundation: Membership on an entity's Board of Directors or advisory committees; Freeline: Research Funding; Takeda: Research Funding; Pfizer: Honoraria, Research Funding; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Indiana Hemophilia and Thrombosis Center, Inc: Current Employment. Zazzali:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd.: Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal